Dr. Eran Perlson, Ph.D.
Department of Physiology and Pharmacology
Research
Dr. Eran Perlson, Ph.D.
Department of Physiology and Pharmacology

The lab is a new multi-disciplinary molecular and cellular neurobiology lab. The lab uses state-ofthe- art single molecule live imaging techniques on neuronal cultures, as well as biochemistry, cell biology and biophysics approaches on mouse model systems to study the role of axonal transport in neurodegenerative diseases, with an initial focus on ALS.
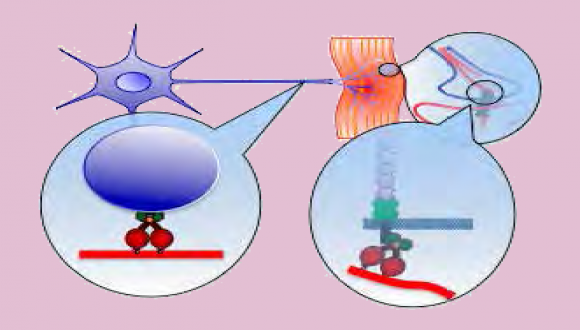
Neuronal survival and proper function depends on cell-cell communication mediated by ligandreceptor mechanisms. During neurodegenerative diseases such as Amyotrophic Lateral Sclerosis (ALS), there is considerable synapse/neuromuscular junction (NMJ) disruption and neuronal cell death. It is non-autonomous processes involve interactions between the neurons to its diverse extracellular microenvironments. The molecular basis for this neuronal dysfunction and death is still poorly understood. One possible reason is alterations in the nature, directed movement and spatial localization of vital extra and intracellular signals.
The long-term research goal of the lab is to understand the vital molecular communications mechanisms between the neurons and its environment. More specifically, we seek to understand the role that retrograde signaling plays in (1) neuronal survival and (2) synapse stability. We believe that our research will generate novel insights into neurodegenerative mechanisms and ultimately, provide a molecular basis for new drugs as well as delivery methods to treat a range of neurodegenerative diseases.
| 2011-2015 | ISF (Israel Science Foundation), The Dual Role of Dynein in GDNF Signaling |
| 2011-2015 | Marie Curie International Reintegration Grants (IRG), Retrogade Signaling. |
| 2013-2016 | Small Molecule Screen for Neuromuscular Junction Maintenance, Rosetrees Trust |
| 2013-2016 | E-Rare-2, European Research Projects on Rare Diseases driven by Young Investigators. Project Coordinator. The Molecular Basis of Neurodegeneration and Muscle Atrophy in ALS. (Co-PIs: Roded Sharan, TAU; Edgar Gomes, U of Paris; Marcus Kruger, Max Planck; Del Bene Fillippo, Ins Curie; Alberto Rodendo, 12th Oct Uni Hospital Madrid) |
| 2013-2018 | Molecular Communication Mechanism of Motor Neuron Survival and Synapse Maintenance, European Research Council (ERC) Starter Grant |
Research
Dr. Moshe Parnas, Ph.D.
Department of Physiology and Pharmacology

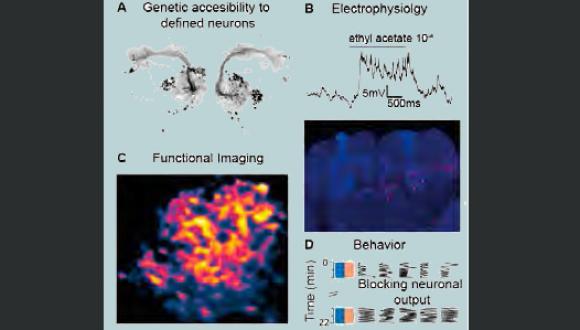
We are exploring the various mechanisms by which neural circuits encode information and support behavior, learning and memory. In addition, we are studying how the connectivity and activity of such circuits and neural networks are affected by molecular mechanisms underlying brain disorders. We use a multidisciplinary approach, with the Drosophila olfaction system as our model system. Our studies incorporate in vivo whole cell patch recordings, in vivo functional imaging, behavior experiments, molecular biology, mathematical modelling and genetics.
Projects in the lab include:
Research
Prof. Illana Gozes, Ph.D.
Department of Human Molecular Genetics and Biochemisty

Our research is characterized by a multi-level approach to the study of brain function, behavior, memory and drug discovery, from molecules to cures. Targeting autism, schizophrenia as well as Alzheimer’s disease and related neurodegeneration and utilizing a multidisciplinary approach, our group investigates different aspects of neuronal plasticity and nerve cell protection, at the molecular, cellular and system level.
A major focus in the laboratory is on nerve structure and transport mechanisms. We have discovered novel families of proteins associated with cross talk among nerve cells and their support cells, including activity-dependent neurotrophic factor (ADNF) and activity-dependent neuroprotetive proteins (ADNPs, with ADNP being a major gene mutated in autism). Small ADNF and ADNP derivatives are in clinical development. The lead compound, davunetide is planned for an advanced Phase II clinical trial with the biotech industry.
Davunetide has previously shown efficacy in several Phase II clinical trials (i.e. in patients suffering from mild cognitive impairment, preceding Alzheimer’s disease and in schizophrenia patients, protecting activities of daily living).
Research
Prof. Hagit Eldar-Finkelman, Ph.D.
Department of Human Molecular Genetics and Biochemistry

Our research is focused on the molecular mechanisms regulating the protein kinase GSK-3 and their implications in human disease. GSK-3 is a central player in diabetes, neurodegenerative and psychiatric disorders, and recently emerged as a promising drug discovery target. We propose that inhibition of GSK-3 should produce therapeutic benefits in treating these disorders. We develop selective substrate competitive GSK-3 inhibitors and evaluate their efficacy and therapeutic effects in relevant in vitro and in vivo systems. So far we could show that our leading compound inhibitors had therapeutic efficacy in CNS disorders models for Alzheimer’s disease, mood disorders, and multiple sclerosis.
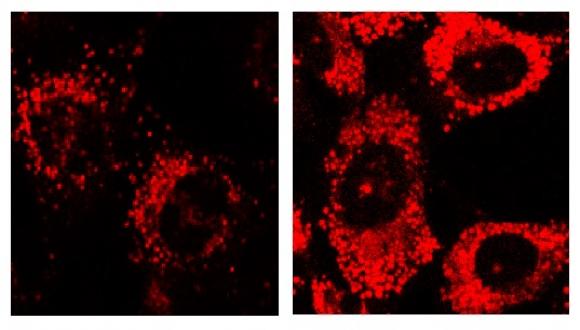
In recent work we identified the lysosome as a GSK-3 target. This implicated GSK-3 as a key player in protein degradation pathways, particularly autophagy ad endocytosis. Research methods combine cell biology, molecular biology and biochemistry disciplines together with bioinformatics and computational biology.
Research
Prof. Ilan Tsarfaty, Ph.D.
Department of Clinical Microbiology and Immunology

Breast cancer is the most common malignant disease in western women. In the majority of cases the cause of death in cancer patients is not the primary tumors, but complications derived from metastases at distant sites. The met proto-oncogene product (Met - a receptor tyrosine kinase) and its ligand, hepatocyte growth factor/scatter factor (HGF/SF), mediate cell motility and proliferation in vitro and tumorigenicity, angiogenesis and metastasis in vivo. Mimp/Mtch2, a mitochondrial carrier homologue cloned in our lab, is induced by Met-HGF/SF signaling and is involved in metabolic and bioenergetic processes. We have previously shown that activation of Met by HGF/SF induces an increase in tumor blood volume in a dose-dependent manner. Mimp/Mtch2 reduces cells proliferation in vitro and tumor growth in vivo. Several anti-Met targeted therapies are in development and some have entered phase III clinical trials.
The goal of our studies is to further understand the role of Met-Mimp/Mtch2 in cancer progression and metastasis, and to develop modalities for personalizing targeted Met therapy. Fluorescent tagged–Met proteins were used to study Met mitogenic effect on cells. Met induced cell motility is mediated by the formation of membrane structures such as ruffles, pseudopodia and blebs. Over expression of GFP-Met WT results in its constitutive activation, cell rounding and detachment, and dynamic nonapoptotic membrane blebbing. Bleb retraction results in numerous membrane microspikes where CFP-Met WT, YFP-actin and membrane markers accumulate.
Expression of Dominant-Negative (DN) YFP-Met alone did not induce any membrane blebbing, and co-expression of CFP-Met WT and YFP-Met DN significantly reduces membrane blebbing. Using confocal based molecular imaging we also show that Mimp/Mtch2 reduces the levels of reactive oxygen species ROS and prevents the HGF/SF induced increase in ROS. Mimp/Mtch2 also reduces the polarization of the mitochondrial membrane potential.